

Vol. 38 (Nº 51) Year 2017. Page 7
Karina Vanelli KOGUTA 1; , Paulo Cesar FLÔRES 2; , Giovana Bomfim de ALCANTARA 3; , Antonio Rioyei HIGA 4
Received: 06/06/2017 • Approved: 28/06/2017
ABSTRACT: The objective of this study was to evaluate the rooting rate and survival effects of minicuttings. Five successive subcultures from mature trees (30 years old) were compared to the control (juvenile cuttings). The statistical design was a randomized block with 8 replications and 6 plants/ plot. The fifth subculture showed the best rooting rate among treatments and no statistical difference in the survival of the cuttings. To evaluate the effectiveness of micropropagation, similar tests were carried. |
RESUMEN: El objetivo de este estudio fue evaluar la tasa de enraizamiento y los efectos de supervivencia de la minicuttings. Se compararon cinco subcultivos sucesivos de árboles maduros (30 años de edad) con el control (esquejes juveniles). El diseño estadístico fue un bloque aleatorizado con 8 replicaciones y 6 plantas/parcela. La quinta subcultura mostró la mejor tasa de enraizamiento entre los tratamientos y ninguna diferencia estadística en la supervivencia de los esquejes. Para evaluar la efectividad de la micropropagación, se realizaron pruebas similares. |
Cryptomeria japonica has durable wood, is resistant to rotting and moisture, as well as to the xylophagous attacks. It is widely used in Japan and in many other Eastern countries for construction, crates and ornamentation (Alves et al.1984, p. 3). Despite being introduced as commercial plantation for more than four decades by the Company Melhoramentos SA, its use is still incipient in Brazil. In southern Brazil its development is similar or even higher to loblolly pine, adapting very well to high and cold regions (Dobner Junior et al, 2013).
According to Carpanezzi e Carvalho (1988, 103 p.), Cryptomeria japonica is originally from Japan temperate region where it is known as "sugi" and occupies approximately 30% of the total forest area of the country. It is one of the most important conifers of Japanese history and its plantations date back several hundred years. In Japan, it occurs naturally between 600 and 1,800 meters of altitude, in a climate characterized by cold winters, with occurrence of snow and moderately hot summers. It is also the most commonly used species in forest plantations in Japan, with great growth in local temperature between 12 and 14ºC, with average annual rainfall above 2,000 mm (Alves et al., 1984).
Although it can be propagated by seeds, vegetative propagation of Cryptomeria japonica is justified by the possibility of maintaining the desirable characteristics. The vegetative propagation or cloning is a technique used to produce plants genetically identical to the parent plant (Hartmann et al., 2002, p. 21). In difficult-to-root woody plant species, the ease of adventitious root formation declines with the age of donors-plant, resulting in a propagation enigma, since desirable characteristics are frequently not expressed until after a plant has reached maturity (Hartmann et al., 2002, p. 307).
As a species which responds well to vegetative propagation through cuttings, implementation and maintenance of clonal gardens with stumps that prove to be capable of producing high-grade rooting seedlings is desirable from an economic standpoint. A clonal mini garden ensures availability of material for cuttings on a large scale. Thus, Cryptomeria japonica cuttings originated from adult trees that had been selected in a provenance test were used. The choice of this material is due to its traceability, creating a germplasm bank for future research. There is however a catch this usage: the cuttings are adult and tissues have high ontogenetic age, decreasing the rooting percentage and increasing the rooting time. A viable alternative is the rejuvenation of these cuttings, maintaining traceability and gaining rooting speed and quality, as for being selected material.
There are numerous features that change during plants maturation. The rooting ability decreases with increasing maturity (Greenwood, Hutchison, 1993, p. 14-33). The maturation of the plant material in woody plants, resulting from the transition from juvenile to adult has received special attention because of morphological, biochemical and physiological changes. However, these changes vary between different species and even within the same species the information is contradictory (Hackett, 1987, p. 216-231).
Several methods have been developed, based on this understanding, to reinvigorate/rejuvenate plants or maintain their juvenility for the purposes of clonal propagation and optimal tree growth. The most common of these are serial propagation, successive pruning and coppicing (Wendling, Trueman and Xavier, 2014). According to the literature, several methods the rejuvenation have been used experimentally and two stand out for their results, serial cutting and micropropagation. About the method serial cutting there is little available material in the literature, there are only articles on the use in Eucalyptus sp. (Eldridge et al, 1994, p. 228-246). It consists of the production of shoots from rooted cuttings. The micropropagation is considered the most efficient technique. However due to technical difficulties with the disinfestation, little is known about its use in rejuvenating species for commercial uses. Studies for Eucalyptus sp. show that the best results are obtained from successive subcultures in vitro (Assis, 1996).
In rooting cuttings of difficult species it would be useful to be able to induce rejuvenation to easily rooted juvenile or transition stage from plants in the mature form. This has been done in several instances by the methods such as serial micropropagation and cuttings. Ready-rooting cuttings can be produced from stock plants that are produced via micropropagation. Rejuvenation of tissue in vitro has tremendous potential to enhance rooting ability. Stock plants derived from micropropagation exhibit certain juvenile/ transition characteristics and produce an increased number of higher rooting, thin-stemmed that conventionally produced stock plants (Hartmann et al., 2002, p. 308).
Protocols based on in vitro plant tissue propagation (micropropagation) have great potential for large scale production of forest species and integrate breeding programs associated with cloning (Harry, Thorpe, 1994, p. 539-560). The most commonly used approach for micropropagation of hardwood for the purpose of cloning is strongly influenced by the genotype, the physiological state of explant age explant, environmental conditions (light, temperature and composition of the culture medium), and especially at concentrations of plant growth regulators (Ahuja, 1993, p. 3-9).
A critical factor in the formation of new shoots from explants in vitro is the source of plant material to be used, and the best results are often obtained with juvenile or rejuvenated material. Shoot meristems, needles, hypocotyls, epicotyls, cotyledon, zygotic embryos, dormant buds, needles beams and lateral buds can be used as explants for shoot induction in conifers (Thorpe, 1984, p. 435-470). In general, the more youthful and rejuvenated for the explants from donor tissue and the more meristem is a good physiological condition, the greater the possibilities of control of morphogenesis in vitro (Hartmann et al., 2002). The most likely causes for the difficulty of rooting are the changes due to aging, which are quite pronounced in conifers (Greenwood, Hutchison, 1993) and the decline in adventitious roots is one of the most frequent events throughout maturation.
The aim of this work is the rejuvenation through cutting and microcuttings collected from Cryptomeria japonica trees with 30 - year-old for the implementation of a clonal mini garden. Due to the availability of material, it was decided to realize the rejuvenation of study via serial cutting and also via micropropagation to compare the two techniques and define the most efficient for this purpose.
The study was conducted in the greenhouse and in the micropropagation laboratory of Forest Improvement Laboratory (LAMEF -UFPR). For this, mini cuttings shoot collected from Cryptomeria japonica trees with 30-years-old selected in provenances test in Rio Negro Experiment Station were used. The experiment was divided into two parts:
Part 1 - serial cuttings:
This part of the study is the comparison of five serial cuttings of adult shoots and juvenile, in order to compare time to rooting and to confirm the rejuvenation of mature cuttings. Treatments used were: treatment 1: one minicutting subcultures; Treatment 2: two minicuttings subcultures; treatment 3: three minicuttings subcultures; treatment 4: four minicuttings subcultures; treatment 5: five minicuttings subcultures and treatment 6 (control): juvenile minicuttings from the clonal mini garden existing in LAMEF.
The design was a randomized block (RBD) with 8 replications and 6 plants/plot. Tubes containing commercial substrate of pine bark and vermiculite base were used. The collection of regrowth occurred at intervals of 60 days. The minicuttings obtained from the cuttings were kept in rooting house with partial control of temperature 25 ± 2°C and moisture content 70-92%. The evaluations were performed at 60 and 90 days after cutting. Survival and the presence of roots were evaluated. The results obtained were later compared with the results obtained via the micropropagation.
Part 2 - micropropagation:
Initially a test was performed for introduction of the adult plant material in vitro. This test is intended to check the degree of contamination and test the disinfection protocol for the explants. To this end they were used 15 micro cuttings C. japonica originated clonal mini garden of LAMEF. The micro cuttings with approximately 1 cm were sanitized by washing with running water for 5 minutes and immersed in 70% ethanol for 5 minutes, followed by immersion in 2% solution (v/v) sodium hypochlorite for 10 minutes under gentle agitation. Then, inside the laminar flow, the explants underwent washing with distilled water and then make inoculated in test tubes containing 10 ml of medium basic MS medium with PVP (polyvinylpyrrolidone) free of growth regulators.
The initial inoculated explants under cultivation in a growth room, in order to quantify possible contaminants. After 7 days they were evaluated and presented 100% of fungal contamination. This material was discarded and a new disinfection protocol was developed using a fungicide in the culture medium. The test was repeated, adding fungicide DithaneÒ (1 mg L-1) to the culture medium and after the establishing an effective disinfestation protocol, the multiplication was performed in vitro explants, with subcultures every 45 days occurring.
The shoots were inoculated in tubes containing 10 ml of MS medium with PVP (0.5%), indole butyric acid (2 mg L-1) and fungicide DithaneÒ (1 mg L-1). The medium was previously adjusted to pH 5.8 and autoclaved. After the inoculation, the cultures were maintained in the growth room with irradiancy around 30 molm-2s-1 in 25 ± 2°C temperature and 16-hour photoperiod. Assessments were 7, 15 and 30 days after the introduction in vitro and have been evaluated contamination, survival, and rooting. The data were tabulated and analyzed using statistical program Assistat.
The data obtained from minicuttings, as shown in Table 1 below, were analyzed using Assistat program was applied to ANOVA and then performed the Tukey test showed a significant difference of 1% for rooting, but not for the survival.

At 90 days there was no statistical difference in survival of C. cuttings japonica, ranging from 52% to 71% (table 1) that is consistent with the literature. Kratz, Wendling and Brondani (2011) found changes from 40% to 70% in the survival of C. japonica cuttings allocating the difference to the different clones used.
In relation to the roots, the fifth subculture (treatment 5) showed the best results when compared to previous subcultures, showing statistical difference at 1%. The rooting percentage of treatment 5 (fifth subculture) showed no statistical difference in treatment 6 which was the control treatment consisting of juvenile cuttings. This result shows that the reduction in ontogenetic age occurred, promoting rooting. Kratz, Wendling and Brondani (2011) using indole butyric acid obtained 22.5% rooting for cuttings derived from trees 9 years old. The data obtained from 32.25% in the fifth subculture proved superior to these numbers, even without the use of growth regulators. For Eucalyptus grandis clones seven minicutting subcultures promoted an increase in the number of roots and provided greater initial root vigor for clones with less rooting potential, suggesting a positive effect of the serial minicutting technique on E. grandis clones for these characteristics (Wendling, Xavier, 2005).
With respect to micropropagation, survival showed a great evolution between the third and fourth subculture as can be seen in Table 2 below, followed by a stabilization in the following subculture. The statistical difference appears between the first and the fifth subculture demonstrates that a significant gain in survival, however, the control treatment 6 (juvenile) was still higher statically than treatment 5, suggesting that would increase the survival rate more subcultures. Ishikawa (1974) won 76% survival at 30 days using adult material (30) after 6 subcultures and 83% survival with juvenile material. These data corroborate the results of this work and demonstrate that the gain in survival after the fifth subculture is still small.
Regarding to rooting, the difference between the first and the fifth subculture was 31.8% with a considerable gain, as shown in Table 2. There was no statistical difference between the control (6) and the fifth subculture, which demonstrates the feasibility of rejuvenating adult material via micropropagation, and 5 subcultures would be sufficient for the rejuvenation of the material. According to Wendling, Trueman and Xavier (2014) the smaller size and lower complexity of the newly-formed plant can result in more-vigorous and less-lignified shoots that have high rooting capacity and root vigour. This data is consistent with the results found by Hine-Gomez (2003) which obtained 49.8% of rooting and higher than those found by Capaldi (2002) which verified 26% of rooting to 45 days. The microcutting provides higher rooting and root vigour for mature clones of Eucalyptus grandis compared with the traditional cutting (Titon et al., 2003). Seven in vitro subcultures are necessary to rejuvenation adult plants of Tectona grandis (Andrade, 2010).
For other species, the most effective means of rejuvenation is subculture in vitro explants, according to Beck, Dunlop and Van Staden (1998), the memory brought to the explant at the time of collection decreases progressively with the in vitro cultures, and the cell programming which is passed to the next subculture. Serial micropropagation has been recommended as a technique to rejuvenation adult trees, but generally the level of rejuvenation not affects all morphological characters similarly.
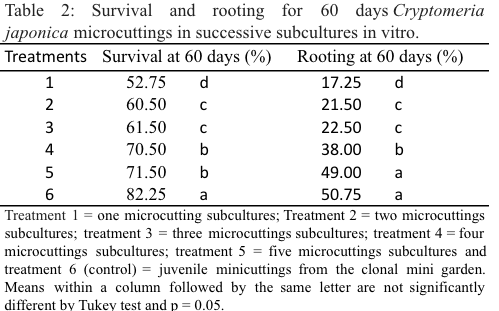
When comparing the results obtained by the two different methods of rejuvenation after 5 successive subcultures, we see that the roots in serial minicutting was 35.25% and micropropagation 49%, a difference of 13.75%. However, it is important to note that the stability of putatively-rejuvenated characteristics has often been questioned and we cannot assume that apparent rejuvenation of one character, like rooting ability, is accompanied by permanent rejuvenation of other traits, like growth rate. The treatments used for phase reversion provide relative rather than absolute rejuvenation (Greenwood, Hutchison, 1993, p. 14-33; Wendling, Trueman and Xavier, 2014).
Despite the higher rooting, plantlets produced by micropropagation, still need to go through the acclimatization phase to the nursery, where this phase usually occurs mortality of individuals. This added to the high deployment and maintenance costs of a laboratory micro propagation, the need for skilled labor, cost of use of plant growth regulators and fungicides, make the serial minicutting is a more favorable option for the production of Cryptomeria seedlings japonica in small and medium scale.
To originate cuttings from adult trees of Cryptomeria japonica rejuvenation through serial minicuttings and micropropagation are viable alternatives. From the fifth subculture on, rooting presents the best results.
Due to the high costs and demands of micropropagation, it is recommended to serially minicutting as adult material rejuvenation method of C. japonica in small and medium scale.
Ahuja, M. R. (1993). Micropropagation a la carte. In: Ahuja, M. R. Micropropagation of woody plants. Kluwer Academic: Dordrecht.
Alves, S. T., Shimizu, J. Y., Higa, A. R., Higa, R. C. V. (1984). Teste de procedência de Cryptomeria japonica em três regiões do Estado do Paraná. Curitiba: Embrapa.
Andrade W. F. (2010). Indução de rejuvenescimento de teca (Tectona grandis L. f) através de enxertia seriada e micropropagação. (Tese de doutorado). Recuperado de http://www.teses.usp.br/teses/disponiveis
Assis, T. F. (1996). Propagação vegetativa de Eucalyptus por microestaquia. In: reunião técnica de propagação vegetativa, SBS: Piracicaba.
Beck, S. L., Dunlop, R., Van Staden, J. (dezembro, 1998). Micropropagation of Acacia mearnsii from ex vitro material. Plant Growth Regulation, 26(3), 149-148.
Capaldi, F. (2002). Avaliação de diferentes fontes de nitrogênio em explantes de Cryptomeria japonica D. Don. "elegans" cultivados in vitro: análises bioquímicas e relações entre reguladores vegetais (Dissertação de Mestrado). Recuperado de www.teses.usp.br/teses/disponiveis/11/11150/tde-30072002-162030/.../flavia.pdf
Carpanezzi, A., Carvalho, P. E. R. (1988). Zoneamento ecológico para plantios florestais do Estado de Santa Catarina. Curitiba: Embrapa Florestas.
Dobner Junior, M., Trazzi, P. A., Higa, A, R., Arce, J. E. (março, 2013). Crescimento de um povoamento de Cryptomeria japonica no sul do Brasil. Scientia Forestalis, 41(97), 39-46.
Eldridge, K., Davidson, J., Hardwiid, C., Wyk, G. V. (1994). Eucalypt domestication and breeding. Oxford: Clarendon.
Greenwood, M. S., Hutchison, K. W. (1993). Maturation as a developmental process. In: Ahuja, M. R., Libby, W. J. Clonal forestry: genetics and biotechnology. Springer-Verlag: New York.
Hackett, W. P. Juvenility and maturity (1987). In: Bonga, J. M., Durzan, D. J. Cell and tissue culture in forestry: general principles and biotechnology. Dordrecht: Martinus Nijhoff.
Harry, I. S., Thorpe, T. A. (1994). In vitro culture of forest trees. In: Vasil, I. K., Thorpe, T. A. (Eds.) Plant Cell and Tissue Culture. Kluwer Academic Publishers: Dordrecht.
Hartmann, H. T., Kester, D. E., Davies Junior, F. T., Geneve, R. L. (2002). Plant propagation: principles and practices (7a ed.). New Jersey: Prentice Hall.
Hine-Gómez, A., Valverde-Cerdas, L. (setembro, 2003). Establecimiento in vitro de Cryptomeria japonica (Taxocidaceae). Revista de Biología Tropical, 51(3-4).
Ishikawa, H. (1974). In vitro formation of adventitious buds and roots on hypocotyl of Cryptomeria japonica. The Botanical Magazine Tokyo, 87, 73-77.
Kratz, D., Wendling, I., Brondani, G. E. (agosto, 2011). Concentrações de ácidos indolbutírico no enraizamento de Cryptomeria japonica. Journal of Biotechnology and Biodiversity, 2(3), 14-21.
Thorpe, T. A., Biondi, S. (1984) Conifers. In: Handbook of Plant Cell Culture - v. 2. Sharp, W. R., Evans, D. A., Ammirato, P. V., Yamada, Y. (Eds.). MacMilIan: New York.
Titon, M., Xavier, A., Otoni, W. C., Reis, G. G. (2003). Efeito do AIB no enraizamento de miniestacas e microestacas de clones de Eucalyptus grandis W. Hill ex Maiden. Revista Árvore, 27(1),1–7
Wendling, I., Xavier, A. (2005). Influência da miniestaquia seriada no vigor radicular de clones de Eucalyptus grandis. Revista Árvore, 29(5), 681-689.
Wendling, I., Trueman, S. J., Xavier, A. (2014). Maturation and related aspects in clonal forestry—part II: reinvigoration, rejuvenation and juvenility maintenance. New Forest, 45, 473-486.
1. Graduanda em Engenharia Florestal, UFPR;
2. Doutorando Engenharia Florestal, UFPR;
3. Professor Departamento de Ciências Florestais, Setor de Ciências Agrárias, UFPR, Email: giobomfim@ufpr.br
4. Professor Departamento de Ciências Florestais, Setor de Ciências Agrárias, UFPR