Espacios. Espacios. Vol. 30 (2) 2009. Pág. 25
Desiree Moraes Zouain * y Marco Antonio Grecco D’Elia**.
Recibido: 20-10-08 - Aprobado: 01-02-09
|
ABSTRACT: |
|
RESUMEN: |
According to FLEURY (2003), the tariff barriers to international trade have been reduced. Considering the tariffs media applied to the industrialized nation’s goods, it used to be 40% in 1947, the year of the GATT - General Agreement on Tariffs and Trade - creation, and becomes about 5% at the end of Uruguay Round and the creation of WTO - World Trade Organization. Now, the trend is to be consolidated near to zero in expressive number of sectors. The protectionist bias, however, was kept through non-tariff measures, among them are the so called “technical barriers”.
Preliminary studies performed by the authors identified that there are important knowledge gaps about the extension and origin of technical barriers and the success factors in overcoming them by producers and exporters, mainly the small and medium Brazilian technology based companies (company based on the development of new products and processes supported by systematic application of scientific and technological knowledge, and the use of advanced and pioneer techniques (ANPROTEC, 2002)).
This study has the following objectives: to identify technical standards, regulations and conformity assessment procedures on European Union (EU), focus on medical devices; to compare the EU practices with the Brazilian’s; to survey small and medium Brazilian producers and exporters of medical devices to EU, searching the difficulties and ways of overcoming them (best practices); and to propose a procedure to identify and overcome the technical difficulties to export medical devices to EU, based on the experiences studied and obtained from the small and medium Brazilian producers.
The international trade was developed in such away that, at the same time, it permits the goods and services trade intensification, it creates restriction and sophisticated controlling mechanisms, sometimes justified considering international rules, sometimes arguable.
These difficulties to exportation have been called by different names: obstacles, impediments or barriers to trade, designations that are used with qualifications like: tariff, non-tariff, technical, among others.
“Technical barriers” to trade occur as result of technical standards utilization and regulations not transparent or that are not based on standards internationally accepted, or even, in result of adoption of conformity assessment procedures not transparent or excessively expensive, as well as inspections excessively rigorous (WTO, 1994).
According to FERRACIOLI (2002), technical requirements, voluntary or compulsory, are used as dissimulated ways of national market protection, becoming an important restriction factor for goods free circulation. However, says the author, the concept of technical barriers is not well understood, being associated with the exporters difficulties to adopt technical requirements.
The booklet of CNI – National Confederation of Industry and MDIC – Ministry of Development, Industry and Foreign Trade of Brazil and AEB – Brazilian Foreign Trade Association (MDIC, 2002) adds: technical barrier constitute technical requirements established to the products or services in target market and suggests a larger definition to technical barriers including the buyers demands (buyer market expectations and requirements that differ from the origin country ones). So this aspect consists in strong difficulty, as long as it has to be overcome to a successful exportation.
Therefore it can be perceived that technical barriers ever existed, although its relative importance has grown in result of the tariff barriers reduction. Many of these technical barriers were not perceived before because of the tariffs high level itself.
Nowadays, according to RICHTER (2000), one of the WTO’s main functions is the management of the current trade barriers and for elimination of discrimination in the international trade, what is, in part, become fulfilled in two agreements: the TBT – Technical Barriers to Trade and the SPS – Sanitary and Phytosanitary Measures. These agreements intent to harmonize the standards and rules referred to environmental protection, public health and consumers’ safety. However, the standards and government regulations are used as a way to protect domestic markets, as long as the tariffs are decreasing and the politic pressure of competitive sectors becomes stronger. The solution adopted was to harmonize these standards and regulations, based on internationally accepted rules.
To LUGGART & SMARTT (2005), the TBT agreement determines that each country member do not impose products standards that are more restrictive than the necessary to the trade, to reach the legitimate targets as environmental protection, consumers’ health and safety. In this way, this agreement disciplines domestic regulations, essential to assure that they are based on legitimate, objective and scientific considerations.
The TBT agreement (WTO, 1994) presents the following definitions:
The European Union established in 1985 the “New Approach to Technical Harmonization”, aiming to turn the standardization process more flexible and speedy. The European Council became more focused on the essential requirements that each product would have to satisfy – referred, in general, to safety, health, and environment’s and consumer’s protection – transferring the definition of technical details to private standardize organizations, as CEN – Standardization European Committee, CENELEC– Electrotechnical Standardization European Committee and ETSI – Telecommunications Standards European Institute. (INMETRO, 1997)
At the same time, was established the concept of “Global Approach to Conformity Assessment”, through which was consolidate the “mutual recognition” principle for foreign technical standards and regulations. According to this principle, any product legally commercialized in a European Union country must be accepted in the others, since it meets the essential requirements, or it is in accordance with the standards considered “equivalents” by the European Council.
In “New Approach” are developed three directives related to medical devices:
In this article it is considered only the second Directive, since the others show particularities that are out of this scope.
According to EUROPEAN COMMUNITY COMISSION (2003), Directive on Medical Devices – 93/42/EEC encloses the medical devices that are not specified in the two other directives. It divides the products in four risk classes:
Class I: low potential risk (example: correction glass lenses);
Class II A: medium potential risk (example: filling material for teeth);
Class II B: high potential risk (example: X-ray equipment);
Class III: critic potential risk (example: cardiac valves).
The Directive on Medical Devices – 93/42/EEC was established on January 1st 1995. Since June 14th 1998, when the implantation period was finished, all medical devices in European Union should be conform to the established requirements and showing the CE mark (CE - Conformité Européene), which means that a producer, or its legal representative, declares that the product is in accordance with every applied standards and it has been submitted to adequate conformity assessment arrangements.
The harmonized technical standards are related on European Community Official Journal, according to Article 5 of Directive 93/42 EEC.
The medical devices regulation in Brazil is in charge of ANVISA – National Health Surveillance Agency of Ministry of Health.
The ANVISA (2007) Resolution number 32, which substitute the Resolution number 444 (August 31st,1999), establishes that the medical devices legal commerce in Brazil is authorized only to devices registered in ANVISA, demonstrating that they are in accordance with ANVISA (2001) Resolution RDC number 56, which defines the “Essential Requirements for Safety and Efficacy of Health Products”, through the conformity certification issued by authorized bodies operating in SBAC – Brazilian System of Conformity Assessment. The “Essential Requirements” are based on the Brazilian technical standards listed on ANVISA (2007) Normative Instruction number 8. To get the registry of ANVISA, the producer have to present the conformity certificate issued by an accredited Certification Body and the tests have to be performed by an accredited laboratory, according to prescriptions established on Medical Devices Conformity Assessment Regulation (INMETRO number 86 – April 3rd, 2006). The accreditation Body is INMETRO – National Institute of Metrology, Standardization and Industrial Quality.
The ABNT – Brazilian Association for Technical Standards – technical standard NBR IEC 60601-1:1997 – Medical Electrical Equipment - Part 1: General requirements for safety, including amendments, is compulsory for electrical equipments under sanitary vigilance. Actually, there are 27 types of electrical equipment which need compulsory certification for registration in ANVISA.
The first edition of General Standard IEC 60601-1 was published in 1977 entitled: “Medical Electrical Equipment Safety – Part 1: General Requirements”.
The third edition of General Standard IEC 60601-1 (2005), according to SCHMIDT (2005), introduces a great change in the philosophy of the medical electrical equipments safety standards, because it combines the product requirements with the process requirements, through the risk management”. The third edition introduces the concept of “essential performance”, referred to the equipment’s operation characteristics that can affect the safety of patients, users or others.
When it is performed this kind of revision, there is a transition period of three years, as usual, to the producers adequate the equipments to new requirements. In European Union this period of time will finish in 2008. In Brazil, this standard has just been translated but it is not issued by ABNT.
The most important change refer to the need of appliance of risk management by the producers, in conformance with “ISO 14.971 – Medical Devices – Risk management application to medical devices”, to determine if additional risks exist, which were not predicted on the product particular standard, mainly those associated to essential performance characteristics.
The standards IEC 60601-1-X (collaterals) and IEC 60601-2-X (particulars) are in the revision process since 2004, to be used with the second edition of the General Standard IEC 60601-1, and in part are inadequate to be used with the third edition.
It can be observed that in the 54 standards of the IEC series 60601 in use, there are 25 ABNT standards (46%) equivalent to the same edition of the IEC correspondent, and 21 of these ABNT standards (39%) were published till year 2000 (included), showing that there was not a standard revision to the correspondent equipments on the world level, and only four (7%) were published by IEC after 2001 (included), referred to the equipments where the Brazilian industry is competitive (cardiac defibrillators, nerve and muscle stimulators, lung ventilators, and recording and analyzing electrocardiographs). There are 16 ABNT standards (30%) equivalents to the edition before the last of IEC correspondent standards that were revised and re-issued at 2005 and after, absolving the new approach defined on General Standard IEC 60601-1:2005. Even more, there are 13 IEC series 60601 standards (24%) to which there are not a correspondent ABNT standard. Of these, three are new collateral standards, published by IEC after 2005, complementary to the third edition of the General Standard, and nine are particular standards of high technology equipments (electron accelerators in range 1 MeV to 50 MeV, therapeutic X-ray equipment operating in the range 10kV to 1MV, gamma bean therapy equipment, automatically-controlled brachitherapy after loading equipment, transcutaneous partial pressure monitoring equipment, radiotherapy simulators, magnetic resonance equipment, peritoneal dialysis equipments, and X-ray equipment to computed tomography) which are not produced by the Brazilian industry.
According to ABIMO – Brazilian Association of the Industry of Medical, Dental, Hospital and Laboratory Articles and Equipment, the Brazilian industry in this area has about 500 companies. The São Paulo State concentrates 77% of Brazilian medical devices producers, 54% in the capital and 23% in the other cities of the State. In the South Region of Brazil there are 11% of the companies and 12% are divided in other States of the country (ABIMO, 2006).
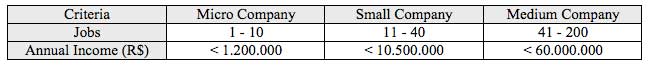
About the companies capacity, according to ABIMO’s estimative adapted to MERCOSUL – Southern Common Market criteria (Table 1), 18% are micro-companies, 36% are small companies, 34% are medium companies, and 12% are big companies. According to ABIMO (2007), this economic sector had income about US$ 3.5 billions, with US$ 442 millions in exportations and creating more than 37 thousand of direct jobs.
Table 1
MERCOSUL stratification criteria for capacity of companies:

Source: Mercosul Resolution GMC nº 90/93 e Mercosul Resolution GMC nº 59/98organizacional.
* University of Sao Paulo. Email: dzouain@uol.com.br
** IPT – Institute for Technological Research. Sao Paulo. Email: magdelia@ipt.br